
Product Definition
Spartina® is a pharmaceutical preparation containing tirzepatide, a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, produced by CinnaGen Company under strict manufacturing standards. Spartina® is manufactured as two forms: sterile pre-filled syringe in Physioject™ (autoinjector), and pre-filled pen for subcutaneous use only.
Spartina® is developed and produced at CinnaGen’s pharmaceutical facilities according to Good Manufacturing Practice (GMP) guidelines. It is intended exclusively for once-weekly subcutaneous administration in adult patients, using designated injection areas (abdomen, thigh, or upper arm), with site rotation recommended.
The active pharmaceutical ingredient, tirzepatide, is synthesized in-house and formulated in a clear, colorless to slightly yellow solution. This guarantees a consistent and high-quality product suitable for healthcare professional prescription use.

Discover Spartina
To improve glycemic control in adults with type 2 diabetes, as an adjunct to diet and exercise
Weight Management
For chronic weight management in adults with obesity, or in those with overweight and at least one weight-related comorbid condition (e.g., cardiovascular disease, dyslipidemia, hypertension, OSA, type 2 diabetes mellitus) alongside a reduced-calorie diet and increased physical activity
Moderate-to-Severe OSA
For the treatment of moderate to severe obstructive sleep apnea in adults with obesity.
Dosing
Spartina® is available as Physioject and pen presentations in the following doses:
Mechanism of Action
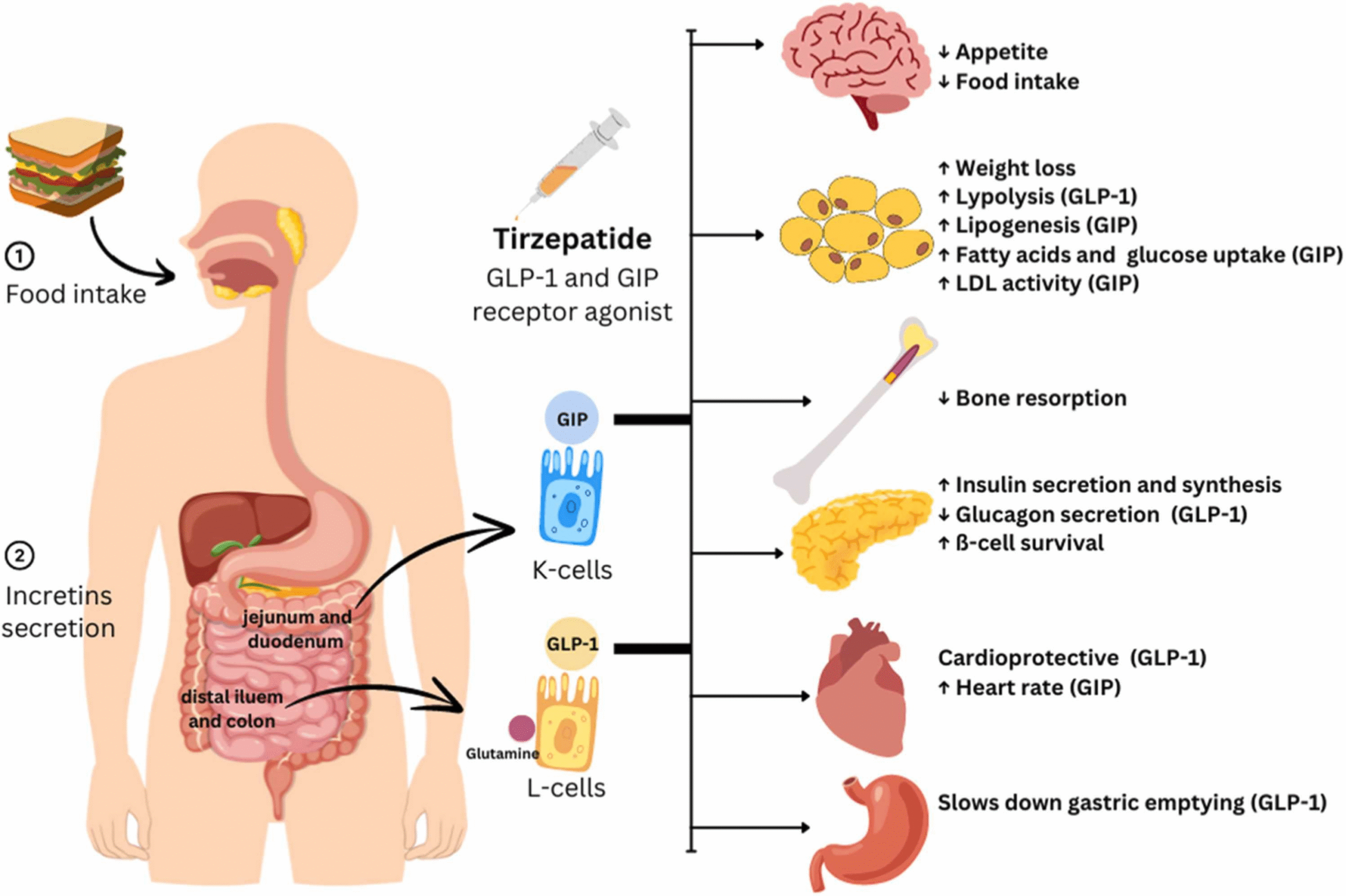
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. It exerts its clinical effects through:
- Enhancement of glucose-dependent insulin secretion
- Reduction of glucagon secretion
- Delay of gastric emptying
- Appetite regulation leading to reduced calorie intake
- Weight loss through fat mass reduction while maintaining lean mass proportionally
Safety Profile Summary Contraindications:
Contraindications
- Personal/family history of Medullary Thyroid Carcinoma (MTC)
- Multiple Endocrine Neoplasia Syndrome type 2 (MEN 2)
- Known hypersensitivity to tirzepatide or its components
Warnings and Precautions
- Acute Pancreatitis: Discontinue if suspected/confirmed
- Hypoglycemia: Higher risk with insulin or sulfonylureas; dose adjustment may be needed
- GI Effects: Nausea, vomiting, diarrhea → risk of dehydration/renal issues; ensure hydration
- Caution in Severe GI Disease: Not studied in severe disorders (e.g. gastroparesis); use with caution
- Diabetic Retinopathy: Monitor patients with active/untreated retinopathy or macular edema
- Anaesthesia Risk: Delayed gastric emptying may increase aspiration risk during procedures
How to Store Spartina®
- Keep Spartina® refrigerated at 2-8°C and out of the sight and reach of children.
- Protect the medication from direct sunlight.
Spartina® is a single-dose product for one-time use only. - Do not shake the device.
- Always check the expiry date before use.
- Never heat or freeze the medication.
- Store Spartina® in its original package until use.
Injection Sites for Spartina®
- The abdomen (at least 5 cm away from the belly button).
- The back and sides of the upper arms (halfway between the elbow and shoulder).
- The front of the thighs (approximately 5 cm above the knee and 5 cm below the groin).
- Assistance from another person may be required if injecting into the upper arm.
- Patients can use the same body area each week but should choose a different injection site within that area.
- If insulin is also being injected, a different injection site should be selected for that injection.
Medical Inquiries & Support
Request a Medical Follow-up
Looking for clinical answers or detailed product information?
Connect with a medical expert from Orchid Pharmed by filling out a short form.
We’ll get back to you quickly.
Speak Directly with Orchid Life
For clinical inquiries or detailed product information, please contact Orchid Life.
+9821 45293
Order a Demo Pen Kit
Demonstration pen kit requests may only be submitted by healthcare professionals who are licensed to prescribe Spartina.
Please complete and submit the demonstration pen kit request form below to begin.
